Medtronic Receives FDA Device Designation for Closed Loop Insulin Pump System
Today Medtronic announced it has received FDA breakthrough device designation for its (PLC) personalized closed loop insulin pump system. It is currently in…

Today Medtronic announced it has received FDA breakthrough device designation for its (PLC) personalized closed loop insulin pump system. It is currently in…
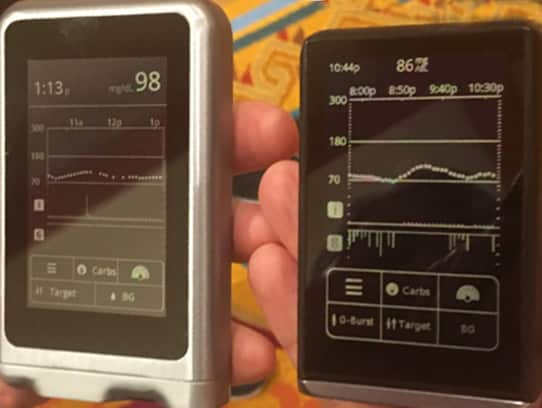
I’ve been touting the bionic pancreas called “The iLet” from Beta Bionics for a while now and it’s launch is…

At the time of this post the Minimed 670g (artificial pancreas) came out about a week ago. The 670g is…
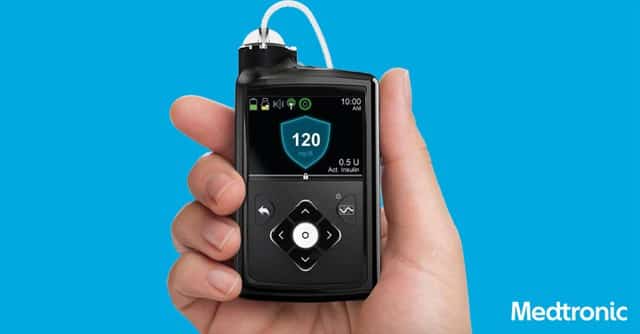
We are all eagerly awaiting the release of the new MiniMed 670G closed loop system for us diabetics. It was approved…
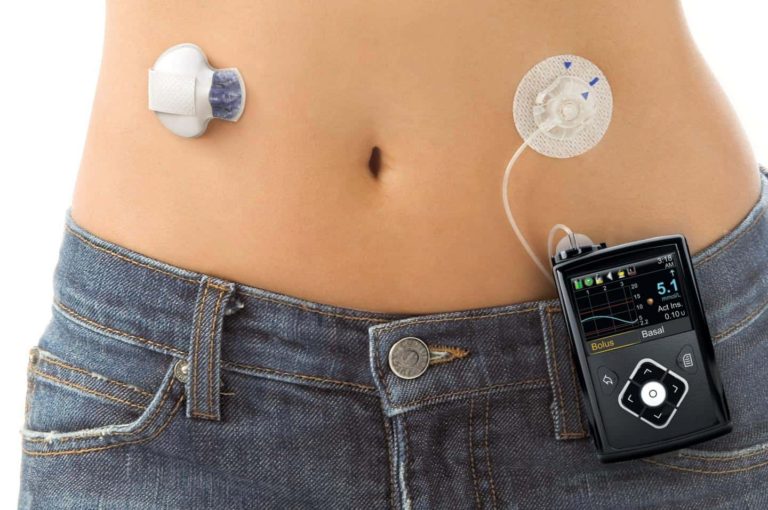
Just a reminder that the MiniMed 670G hybrid closed loop System will be available to the public in the spring…
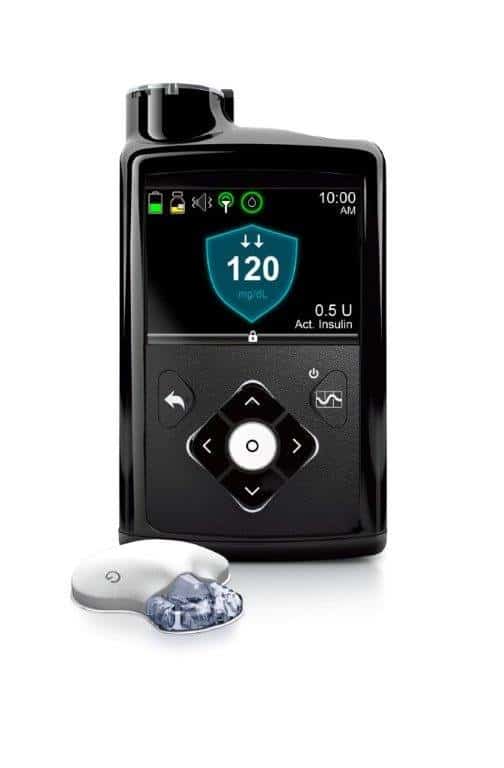
Medical device maker Medtronic has won Food and Drug Administration (FDA) approval for the MiniMed 670G device. The “hybrid closed…

I’m a type 1 diabetic that uses the Continuous Glucose Monitoring System (CGMS) by Medtronic. I use the MiniMed 530G…