Beta Bionics Announces FDA Clearance of the iLet Bionic Pancreas
Previously Published on NewsWire.com — Beta Bionics, Inc. — a medical technology company focused on diabetes — announces FDA 510(k) clearance and the…
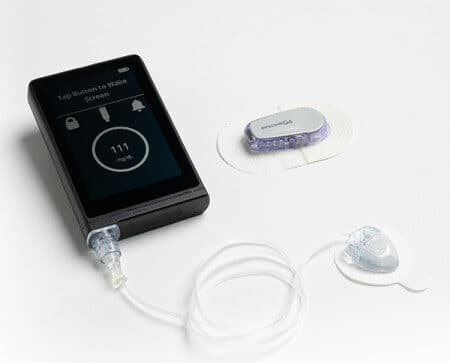
Previously Published on NewsWire.com — Beta Bionics, Inc. — a medical technology company focused on diabetes — announces FDA 510(k) clearance and the…

Today Beta Bionics was granted FDA breakthrough device designation for the iLet bionic pancreas system. The designation was granted in all…
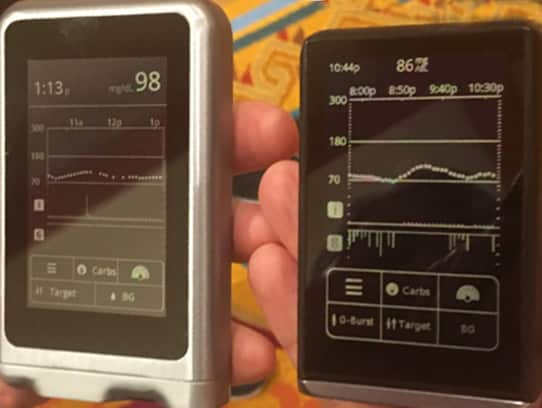
I’ve been touting the bionic pancreas called “The iLet” from Beta Bionics for a while now and it’s launch is…