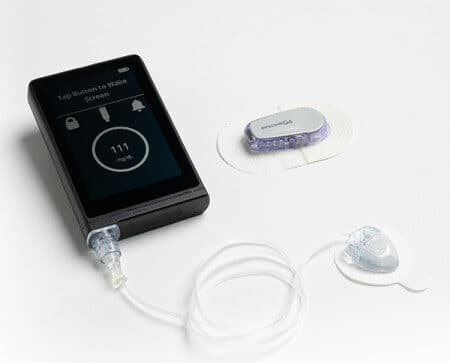
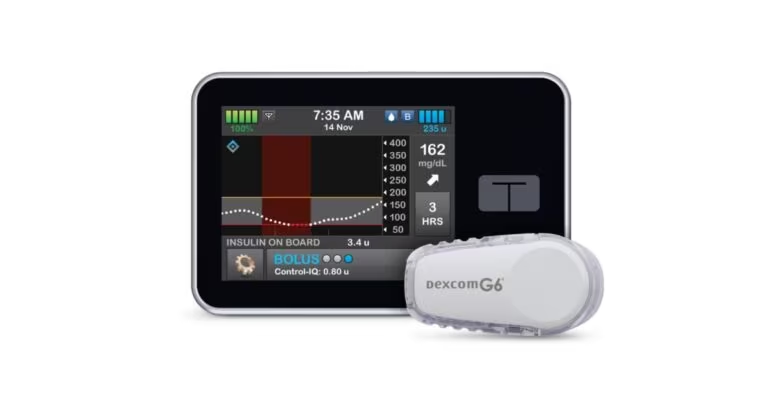
The Benefit of CGMs and Insulin Pumps, How To Best Cover The Cost
To live a life without worry is priceless. Unfortunately, the reality is life all too often is rife with worry and there is a price put on both. This can be especially true for those living with diabetes. The label comes with assumed hindrances, sacrifices, and inflated cost. CGMs and insulin pumps can be very…