Beta Bionics Announces FDA Clearance of the iLet Bionic Pancreas
Previously Published on NewsWire.com — Beta Bionics, Inc. — a medical technology company focused on diabetes — announces FDA 510(k) clearance and the…
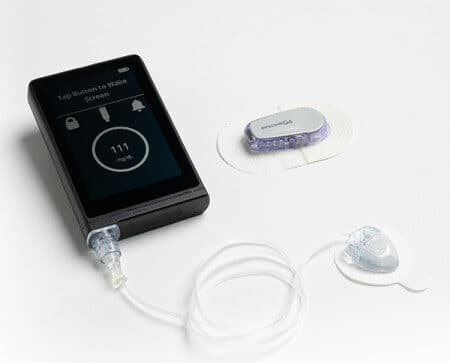
Previously Published on NewsWire.com — Beta Bionics, Inc. — a medical technology company focused on diabetes — announces FDA 510(k) clearance and the…
Previously Published on diaTribe.org – Impressive results from a trial for an up-and-coming artificial pancreas showed that users spent an average…

Previously Published on StatNews – The iLet bionic pancreas closed-loop insulin delivery system provides good glucose control without the need for the…

Today Beta Bionics was granted FDA breakthrough device designation for the iLet bionic pancreas system. The designation was granted in all…

Beta Bionics announced a pivotal bihormonal iLet home trial using both dasiglucagon and insulin in the aforementioned bionic pancreas. The iLet (dual-hormone pump…

Bionic Pancreas Critical Hurdle! Beta Bionics recently won $12 million in supplemental funding from the National Institutes of Health (NIH)…

The Bionic Pancreas called “iLet” by Beta Bionics. The only fully integrated, fully automated, bihormonal bionic pancreas. The device uses both,…

The Artificial Pancreas is a smart idea and does show promise in controlling blood sugars for type 1 diabetics who…