NexImmune Explores AIM Direct Injection Technology in T1D
Previously Published on Biospace.com – Neximmune, Inc. (Nasdaq: NEXI), a clinical-stage biotechnology company developing a novel approach to immunotherapy designed to orchestrate a targeted immune response by directing the function of antigen-specific T cells, today announced a collaboration with Yale University’s Department of Immunobiology. The collaboration will focus on the use of NexImmune’s direct injection, artificial antigen presenting cells (AIM INJ) with regards to the regulation of autoimmune diabetes (Type 1 diabetes). Dr. Kevan Herold, Deputy Director of Yale Center for Clinical Investigation and Co-Director of the Yale Diabetes Center will be the principal investigator.
“We are excited to enter into this collaboration that will explore our next-generation, direct-injectable artificial antigen presenting cell platform for autoimmune diseases,” said Dr. Jerry Zeldis, Executive Vice President, R&D of NexImmune. “Our goal with Dr. Herold is to advance novel treatments that could potentially reverse the course and prevent type 1 diabetes by targeting the auto-reactive T cells that cause this disease.”
“By directly targeting the auto-reactive T cells that are known to be a mediator of pancreatic beta cell destruction, we can potentially develop a transformative therapy for patients suffering with autoimmune diabetes. Working with NexImmune allows us to explore a very compelling technology to impact this life-long and difficult to control disease,” stated Dr. Herold.
NexImmune AIM Direct Injection Technology Clinical Trials
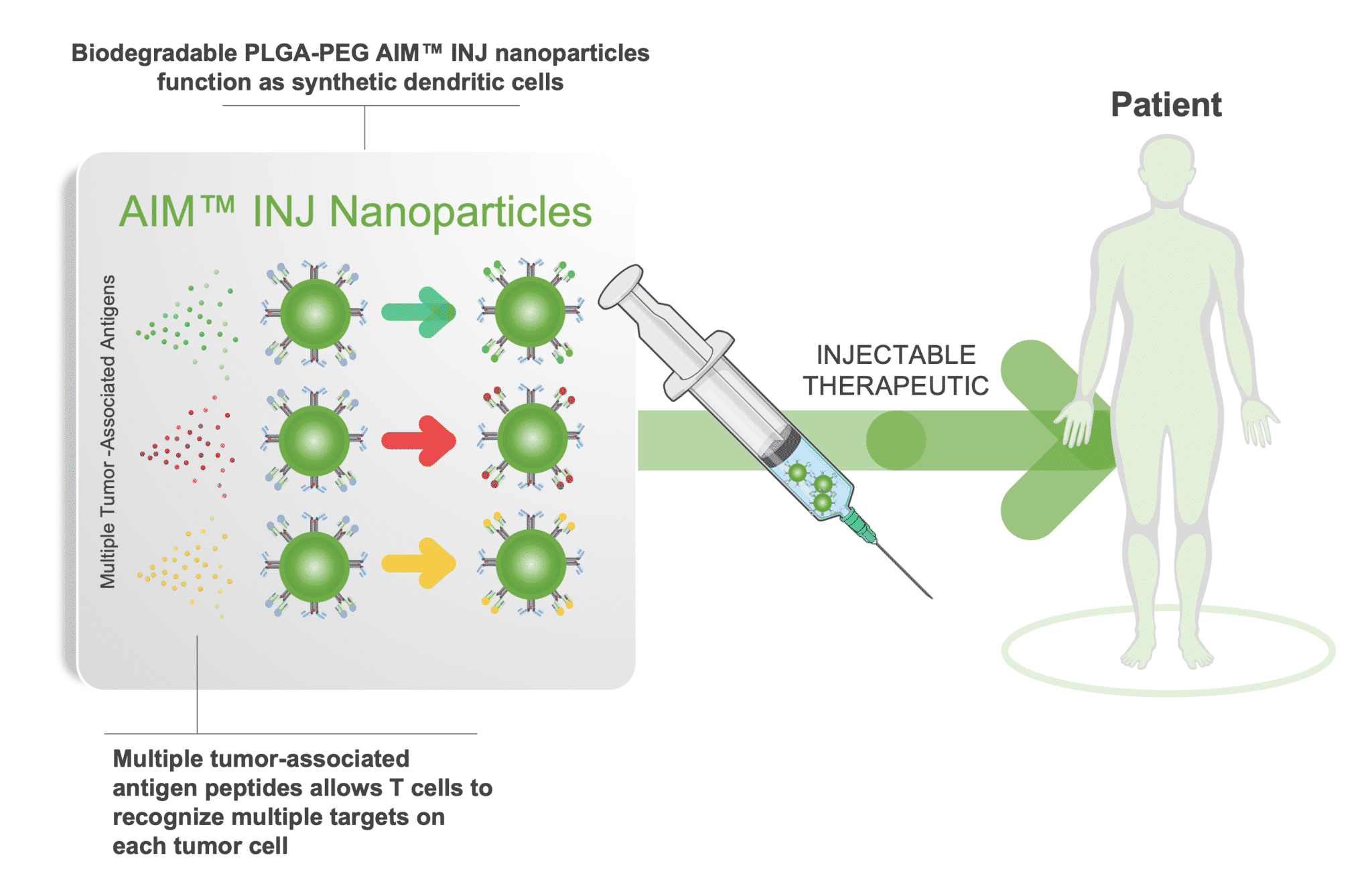
NexImmune is a clinical-stage biotechnology company developing a novel approach to immunotherapy designed to employ the body’s own T cells to generate a specific, potent, and durable immune response. The backbone of NexImmune’s approach is a proprietary Artificial Immune Modulation (AIM™) nanoparticle technology platform. The AIM technology enables NexImmune to construct nanoparticles that function as synthetic dendritic cells capable of directing a specific T cell-mediated immune response. AIM constructed nanoparticles employ natural biology to engage, activate and expand endogenous T cells in ways that combine anti-tumor attributes of antigen-specific precision, potency and long-term persistence with reduced potential for off-target toxicities.
NexImmune’s two lead programs, NEXI-001 and NEXI-002, are in Phase 1/2 clinical trials for the treatment of relapsed AML after allogeneic stem cell transplantation and multiple myeloma refractory to at least 3 prior lines of therapy, respectively. NexImmune is also developing new AIM nanoparticle constructs and modalities for potential clinical evaluation in oncology and in disease areas outside of oncology, including autoimmune disorders and infectious disease.
Previously Published on Biospace.com
More promising research for t1d.