Breakthroughs in Pancreatic Cell Replacement: ViaCyte Interview
Previously Published on Diabetes Daily ViaCyte Interview By Christine Fallabel – I recently had the opportunity to sit down with Manasi Sinha Jaiman, M.D., M.P.H., Vice President of Clinical Development, and Mark Daniels, Senior Director of Clinical Development, of ViaCyte, “a regenerative medicine company focused on delivering novel stem cell-derived cell replacement therapies as a functional cure for all type 1 diabetes and a next-generation treatment for insulin-requiring type 2 diabetes.”
They have amazing things coming down the pike, so I was super excited to speak with them:
Tell our readers about ViaCyte. What’s the company’s mission and story?
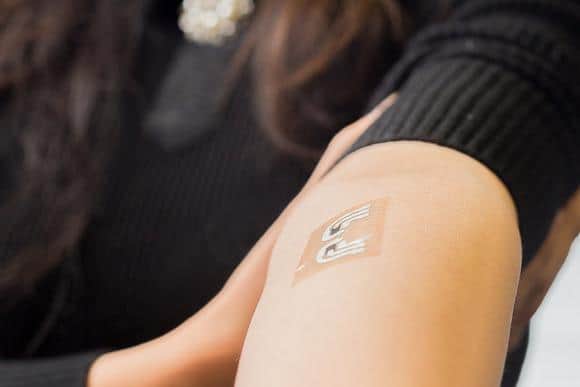
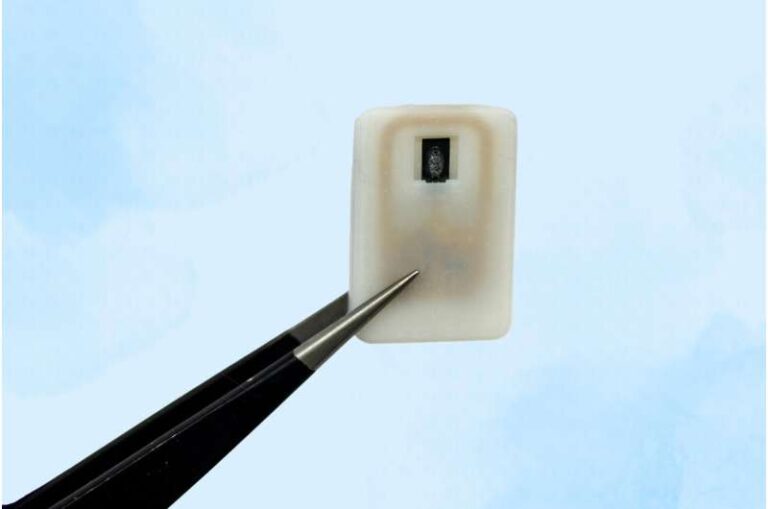
Dr. Jaiman: ViaCyte is at the forefront of regenerative medicine approaches to develop a functional cure for type 1 diabetes (T1D). We have cell replacement therapies for pancreatic islet cells contained in a small retrievable pouch implanted under a patient’s skin.
The therapy is designed to enable insulin and glucagon (the counter-regulatory hormone that treats low blood glucose) production with the implanted cells to effectively control blood glucose levels, decrease the risk of hypoglycemia, and mitigate short-term and long-term diabetes-related complications for patients.
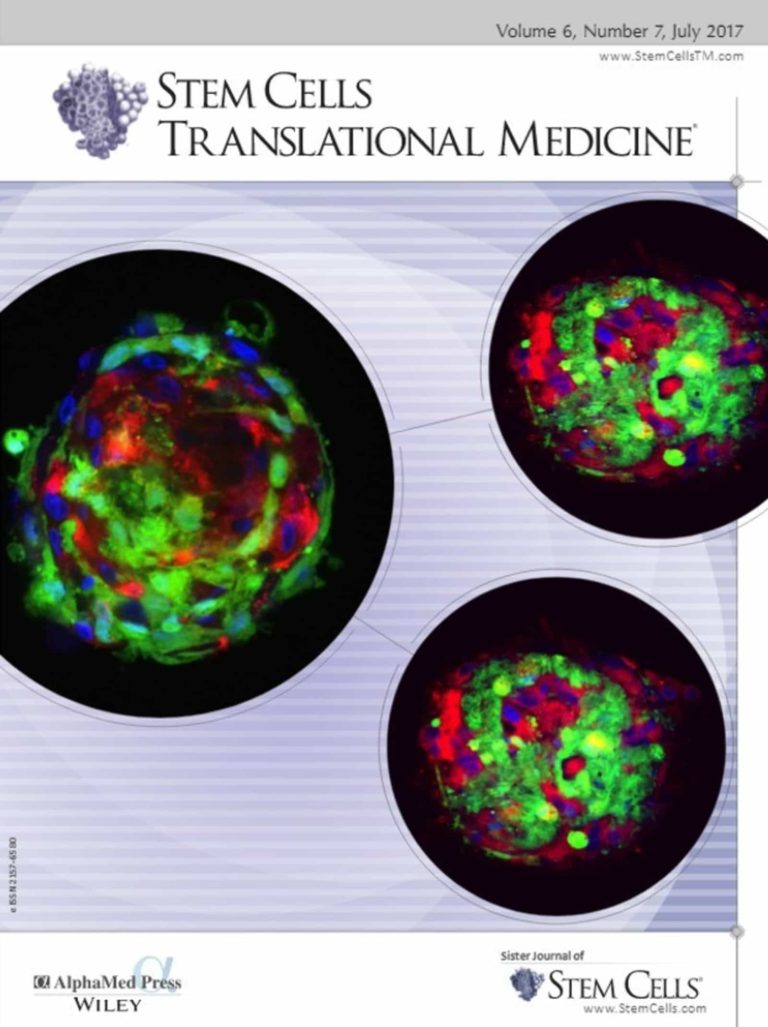
What is exciting is that ViaCyte is the first company to advance human stem cell-derived islet cell replacement therapy capable of producing insulin in the clinic, backed by two decades of research and expertise.
We are further augmenting our therapies by optimizing both the delivery device and the cells through collaborations with industry leaders, including W. L. Gore & Associates (the makers of GORE-TEX) and CRISPR Therapeutics.
Our mission is to develop cell replacement therapies offering long-term treatment to decrease the burden of the constant management needed with T1D.
What led you to your work at ViaCyte?
ViaCyte interview continued:
Dr. Jaiman: A significant focus of my medical career has been the integration of technology and medicine to advance treatments that can change the paradigm of diabetes disease management.
My experiences both in research and actively seeing patients with T1D have given me first-hand experience in seeing the daily work required to achieve any form of glycemic control as well as the burden patients and families face from complications from hypoglycemia or DKA.
I have also been able to see the importance of innovative approaches in addressing their needs. When I looked at the ViaCyte technology, I immediately saw great potential in their regenerative medicine approach to lessen the burden of disease and improve quality of life.
Mr. Daniels: Throughout my time in the industry I have been extremely fortunate to be able to work with innovative companies advancing potential, game-changing therapies for difficult-to-treat diseases. I was attracted to ViaCyte because the bar is set high; ViaCyte is looking to develop functional cures for diseases and is not just treating symptoms.
I also believe in the potential of cell replacement therapies and that these will be a meaningful new chapter in the advancement of medical treatments.
In ViaCyte, I have also found a team of extremely gifted and dedicated scientist-coworkers who are all generous with their knowledge and completely aligned in the mission to deliver a functional cure to the type 1 diabetes community.
It is exciting to be the first company to evaluate human stem cell-derived islet cell replacement therapy for its potential to functionally cure type 1 diabetes in the clinic.
What exciting new developments is ViaCyte currently working on?
ViaCyte interview continued:
Dr. Jaiman: Currently, ViaCyte has two clinical cell replacement therapy candidates. First, VC‑02 PEC-Direct is a treatment comprised of pancreatic islet cells in a pouch designed to allow blood vessels to enter the device and directly interact with the implanted cells to produce insulin and glucagon.
This treatment candidate is targeted for those with high-risk type 1 diabetes (hypoglycemia unawareness) able to tolerate immunosuppression.
In contrast, our groundbreaking VC‑01 PEC-Encap device is an advanced treatment comprised of pancreatic islet cells in a pouch that fully encapsulates the cells preventing immune cells from interacting with the implanted cells, which eliminates the requirement for immunosuppressants.
We are collaborating with W. L. Gore & Associates to optimize their innovative membranes which encapsulate the cells in our implanted devices. We expect to share clinical data in the second half of 2021.
Anything new in the pipeline that people with diabetes should be especially excited about?
ViaCyte interview continued:
Dr. Jaiman: One of the challenges with cell replacement therapies is to protect against adverse reactions and rejection of implants by the body’s immune system, which serves as a defense mechanism against foreign bodies.
In collaboration with CRISPR Therapeutics, we are employing gene-editing technology to engineer cells to avoid recognition by the immune system. Our partnership is focused on advancing gene-edited allogeneic stem cell-derived therapies from discovery through commercialization with the goal of developing a potential next-generation functional cure for all insulin-requiring type 1 and type 2 diabetes.
Mr. Daniels: With our preclinical candidate, VCTX210 PEC-QT, pancreatic islet cells would be in the same pouch as PEC-Direct, allowing the implanted cells to interact directly with blood vessels, an approach intended to enable robust and consistent engraftment.
Yet by designing the cells to be immune-evasive through CRISPR Therapeutics’ gene editing we would expect to eliminate the need for immunosuppressants as are required with PEC-Direct. We look forward to sharing more about this unique program in the future.
Where do you envision ViaCyte and people’s lives affected by diabetes in five years? Ten years?
ViaCyte interview continued:
Mr. Daniels: This year marks the 100th anniversary of the development of therapeutic insulin to regulate blood glucose, yet dependency on tedious insulin injections are still a common course of treatment for many living with type 1 diabetes.
Within the next five years, we envision delivering significant progress in later clinical-stage studies with increased time in range, reduction in hypoglycemic events, and reduction in (or elimination of) the need for insulin injections in patients following our cell replacement treatments as we move toward making these therapies more widely available.
Dr. Jaiman: Within five years, we expect to be moving through the final phases of our regulatory process for our human stem cell-derived islet cell replacement therapy enabling availability more broadly for patients with type 1 diabetes.
It is our hope that within a decade, cell replacement therapy will offer longer-term treatment, easing the burden of constantly monitoring blood glucose. A functional cure will no longer be a dream, rather, a reality.
Is the ever-elusive cure on the horizon? A functional cure?
ViaCyte interview continued:
Dr. Jaiman: Yes, we believe a functional cure is on the horizon!
ViaCyte is focused on advancing cell replacement therapies toward a functional cure with a combination of implanted cells and device engineering.
This cell replacement therapy could represent insulin production protected from the immune system in a way that totally mitigates the underlying disease. Our technology is designed to safely implant the missing cells that make insulin and glucagon – that’s the breakthrough that gets us to the functional cure.
How can people with diabetes get involved or learn more?
Mr. Daniels: As ViaCyte is advancing novel treatments for type 1 diabetes, our team has been very fortunate to collaborate with multiple incredible research and advocacy organizations, including the Juvenile Diabetes Research Foundation (JDRF), Beyond Type 1, and California Institute for Regenerative Medicine (CIRM).
These organizations are focused on education and support for finding a cure for diabetes with resources for both patients and researchers on their websites. A great resource is the website www.clinicaltrials.gov – by typing “ViaCyte” into the search window, you can find more details regarding our ongoing clinical trials.
This includes details about the entry criteria to participate as well as the geographical locations of the sites (to find the one closest to you) and contact details necessary to reach out and connect with the study site team to learn more about what is involved in the study participation.
Anything else you’d like to share?
ViaCyte interview continued:
Dr. Jaiman: Insulin treatment has largely transformed type 1 diabetes from a fatal illness to a chronic one, yet it is not a cure. At ViaCyte we recognize the long journey in the evolution of diabetes management, and we are keen to deliver a solution that offers real hope for a functional cure for type 1 diabetes.
Every single member of our team is passionate and dedicated to this endeavor. Managing diabetes can be difficult at any time, however, this past year has highlighted the need for accelerating therapeutic advancements to help reduce COVID-associated morbidity and mortality in the vulnerable population with diabetes.
With the pandemic still ever-present, we believe our mission of realizing a functional cure is even more critical for patients as they navigate living with a chronic disease during this very trying time. Our leadership team is wholly focused on improving patient care with an eye to the future.
Mr. Daniels: I am very appreciative of the Diabetes Daily team for providing this forum to connect to their community. The stories of family member’s and loved one’s experiences (including those of some of our own coworkers) with T1D resonate clearly within us and fuel our motivation behind the work we do.
It is only through the support of the T1D community and especially the valued study participants that we are able to advance this important research.
We are proud to be part of the biotech and biopharma community advancing some of the world’s most promising medical devices and therapeutic treatments. We look forward to sharing more details regarding the value of these treatments in the clinic.
Previously Published on Diabetes Daily ViaCyte Interview By Christine Fallabel