Beta Bionics granted FDA Breakthrough Device Designation for iLet Bionic Pancreas System
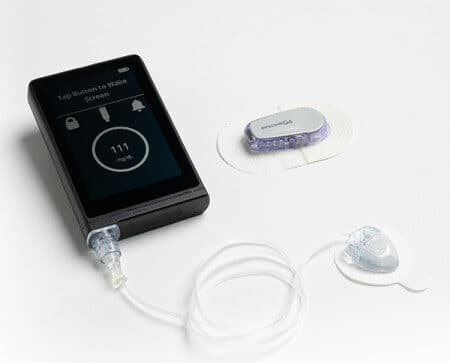
Today Beta Bionics was granted FDA breakthrough device designation for the iLet bionic pancreas system. The designation was granted in all configurations of the iLet (insulin-only, glucagon-only and bihormonal). The meaning of FDA breakthrough device designation is to provide patients and health care providers with timely access to medical devices by speeding up their development, assessment, and review. This is amazing news for people with type 1 diabetes.
The iLet is the world’s first bionic pancreas system that autonomously controls blood sugar in people with type 1 diabetes as well as other conditions. Read full article here!